© 1984 Elsevier Science Publishers B.V.
Uvcitis update
K.M. Saari,editor
Frequency Of Histocompatibility Antigen Bw 51 And Antiguinea Pig Lip Antibody In Behcet Patients And Their Relatives
(1) ANTONIO G. SECCHI, SALVATORE PERRONE, (2) UMBERTO FAGIOLO, AMELIA RUFFATTI, (3) PIER LUIGI MATTIUZ, - CARLO CONIGHI
(1) Department of Oihthalmology, Research Unit for Ocular Allergy and Immunology, and (2) Institute of Internal Medicine, University of Padova, School of Medicine, 35128 Padova (Italy),(3) Institute of Medical Genetics, University of Ferrara, School of Medicine, 44100 Ferrara (Italy)
It is well known that there is a close association between Behcet's disease and the histocompatibility antigen HLA-B5. Recent studies have also demonstrated that the disease is mainly linked to a split portion of HLA-B5, HLA-Bw 51 (1). It has also been demonstrated that there is an immune reaction to oral mucous membrane antigens in Behcet patients, and that during the course of the disease there is a cell-mediated immunity to antigens from the oral mucosa (2), a cell-mediated cytotoxicity for cultured oral epithelial cells (3), and the presence of circulating antibodies to oral mucous membrane antigens (4). Whether these findings are primary pathogenic determinants or only epiphenomena subsequent to the oral ulcerations is, however, still uncertain.
In a series of 10 patients with complete Behcet's disease and in 47 of their first and second degree relatives, we studied the frequency of HLA-Bw51 and the presence of circulating anti-guinea pig lip antibody (AGPLA). Guinea pig lip is the best antigenic substrate for fixation of heterologous anti-mucous membrane antibodies. Organ and non-organ-specific autoantibodies were also studied in both populations.
Methods
- Selection of patients and controls.
We examined 10 patients with the complete form of Behcet's disease, 47 of their relatives, and as control groups 245 healthy individuals for HLA typing and 25 for circulating anti-guinea pig lip antibody. - Anti-guinea pig lip antibody (AGPLA), organ- and non-organ-specific autoantibodies
Antibodies to guinea pig lip were detected by the classical indirect immunofluorescence technique, using unfixed cryostat sections of guinea pig lip and fluorescein-labeled goat anti-human immunoglobulins. The identification criteria proposed by Michelson and Chisari were used. The sera were first analyzed at 1:10 standard dilution and in each positive case the end point of titration was recorded.
Antibodies to nuclei, smooth muscle, mitochondria, microsomes, ribosomes, reticulin and thyroid microsomes were determined by the indirect immunofluorescence method on unfixed cryostat sections of rat stomach, kidney and liver and human thyroid at a 1:10 serum dilution.
Antibodies against native DNA were tested by the indirect immunofluorescence method, using Crithidia Luciliae as substrate. - HLA typing
HLA typing was performed on peripheral lymphocytes according to the technique proposed by the IX International Histocompatibility Workshop; NIH standards were adhered to.
Results and Discussion
None of the patients' relatives showed any present or past signs of even subclinical Behcet's disease.
Table 1 shows the data on the frequency of HLA-BW51.
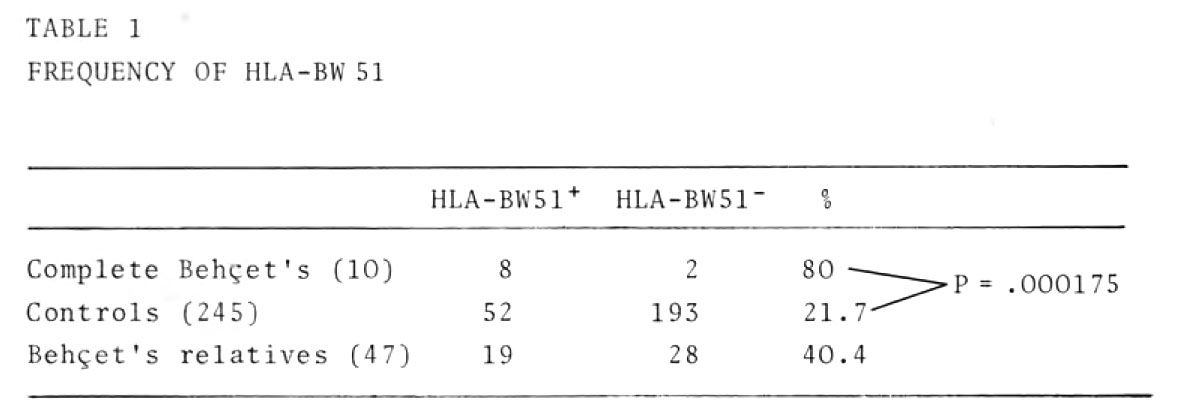
Our finding confirms in the Italian population Behcet's disease is clesely correlated with the antigen HLA-BW51, which was also present in a high percentage of the patients' relatives.
Table 2 summarizes the data on the frequency of antimucous membrane antibodies
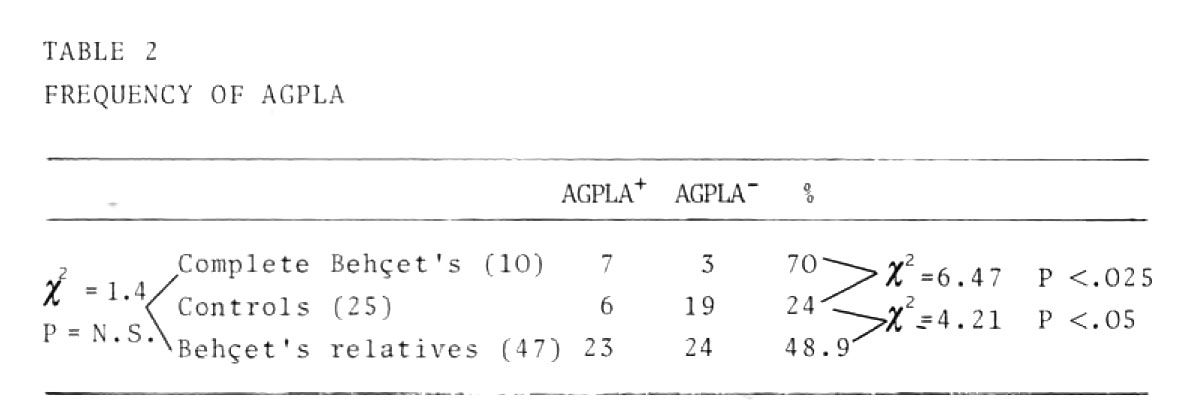
The frequency of anti-guinea pig lip antibody in the patients with complete Behcet's, and in the relatives, was significantly higher than in the control; no statistical difference was found between the incidence of the antibody in the patients' relatives group in the complete absence of any clinical mucous membrane ulceration, and its incidence in the patients' group. This finding suggests that in a so far healthy population with a genetic predisposition to Behcet's disease, the appearance of antibodies against mucous membrane antigens may be considered a primary pathogenetic determinant rather than a consequence of mucous membrane ulceration. It may also be possible, however, that these antibodies appear consequently to primary subclinical mucous membrane damage. Very low levels of circula ting AGPLAs, are, in fact, present in these populations.
Table 3 shows the data on the presence of these antibodies at dilutions of over 1:80
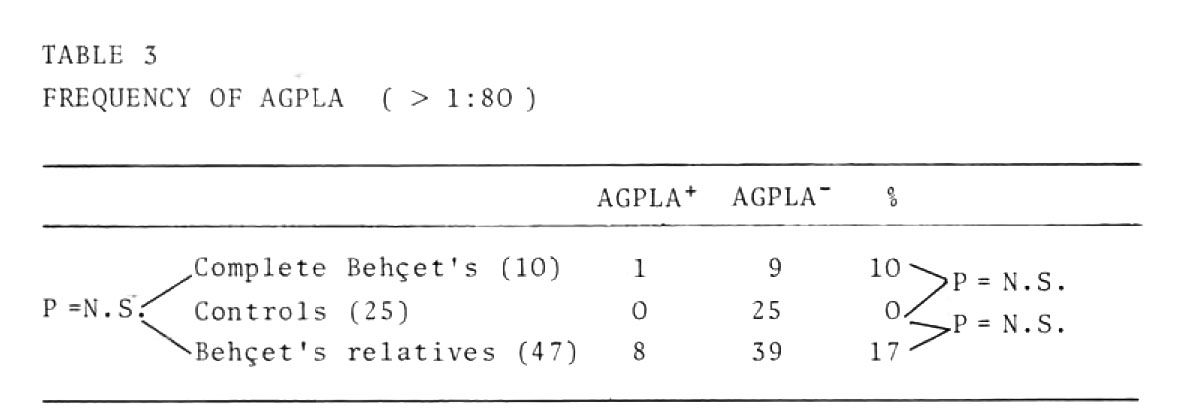
In our opinion these findings cast doubt on the pathogenic role of these antibodies. Their presence in a population genetically predisposed to Behcet's disease without any clinical sign of the disease (patients' relatives) cannot, however, be considered casual.
Data obtained by evaluating the correlation between the presence of AGPLA and HLA-BW51 are shown in table 4.
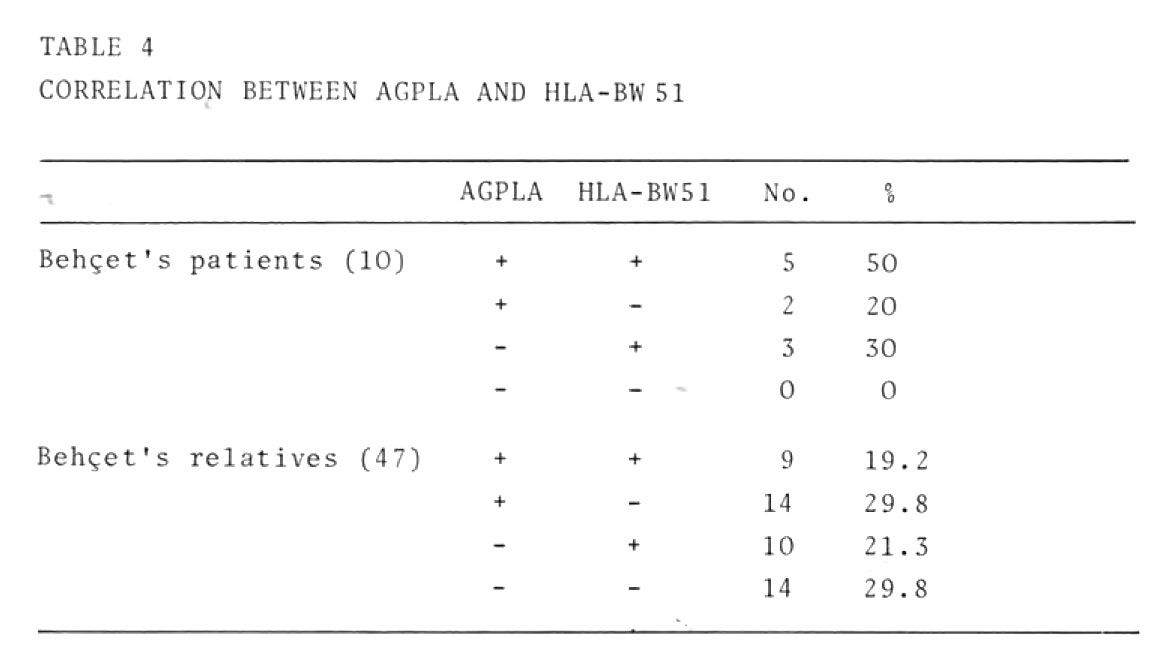
Our patient population was too small to allow definitive conclusions to be drawn, but the percentage (50%) of patients with total positivity is substantially higher than the percentage of relatives (20%) with full positivity. Moreover no patient had total negativity, whereas total negativity was found in one third of their heal thy relatives. Further studies are r quired to establish whether or not there is a correlation between these two markers.
Table 5 summarizes the data concerning the presence of circulating autoantibodies in the populations we have tested.

Our findings confirm that both HLA-BW51 and anti-guinea pig lip antibodies are important "markers" in Behcet's disease; the correlation between them and their actual involvement in the pathogenesis of this disease, however, is yet to be discovered.
References
-
Ohno S, Ohguchi M, Hirose S, Matsuda H, Wakisaka A, Aizawa M (1982) Arch Ophthalmol 100:1455-1458
-
Lehner T (1969) J Pathol 97:481-494
-
Rogers RS, Sams WM, Shorter RG (1974) Arch Dermatol 107:361-363
-
Michelson JB, Chisari FV (1982) Surv Ophthalmol 26:190-203