J. Trace Elem. Electrolytes Health Dis.
Vol. 3, 1989, pp. 209-212
Stable hyperzinchemic condition in patients with behcet's syndrome. Lack of correlation with in vitro cellular immune response capacity
A. Balboni, L. Melchiorri, (1) R. Vecchietti, (2) S. Perrone, A. Sensi, M. Venturi and O.R. Baricordi lstituto di Genetica Medica
(1) Dipartimenro di Chimica, Università di Ferrara,
(2) Clinica Oculistica, Università di Padova, Italy
Summary
We have observed a significant increase of zinc concentration in plasma and erythrocytes in a group of Italian Behcet patients, evidencing a rare hyperzinchcmic condition associated with the disease. The increased zinc level observed does not show any relation to the presence of therapy or with the impairment of the hlastogcnic response to HSVI, a specific cellular defect in Behcet disease, confirmed in the present study. The possible biological meaning or these observations is briefly discussed.
Keywords
Zinc, hyperzinchemia, Behcet's disease, cellular immune response.
Introduction
Behcet's disease (BD) is a multisystem disorder characterized by a multifocal vasculitis which has its clinical hallmarks in: (a) recurrent aphtous ulcerations of oral mucous membranes coming in crops lasting one to two weeks; (b) skin lesions, including erithema nodosum, superficial thrombophlebitis, folliculitis and acne-like lesions, and cutaneous hypersensitivity; (c) ocular symptoms (Figure I) such as iridocyclitis, featuring transient hypopyon iritis and posterior synechiae, chorioretinitis, and their sequels; (d) genital ulcers or painful punched-out lesions (1).
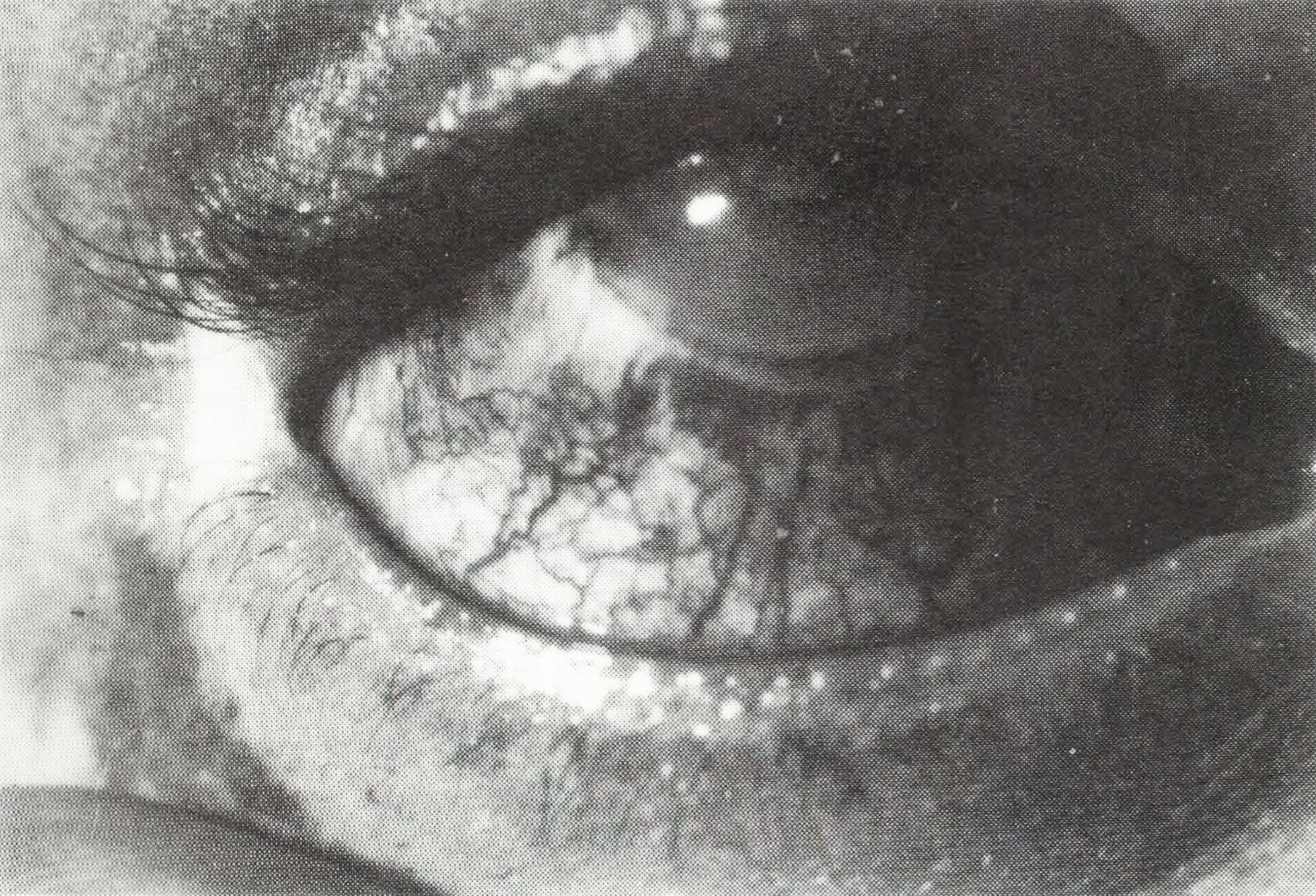
Figure 1. Characteristic ocular lesions of BD: iridocyclitis and episcleritis.
Minor criteria include arthritis, intestinal ulcerations, epididymitis, thrombophlebitis and neuropsychiatric symptoms. The complete type of BD requires the four main symptoms to appear some time during the clinical course, whereas the incomplete type requires the three major criteria to appear some of the time, or that recurrent hypopyon iritis or retinitis is present, accompained by one other major criterion (1).
Although the etiology of the disease is still unknown and both viral and autoimmune hypotheses have been put forward, several findings suggest a cell-mediated immune pathogenesis. The immunological derangement of the disease is evidenced by the presence of immune complexes in serum (2), the imbalance of the T-cell subsets (3) and the specific impairment of the lymphoproliferative response to Herpes Simplex Virus type 1 (4). Furthermore, HLA B51 antigen has been shown to be strongly associated with the ocular type of the disease (5, 6), suggesting the involvement of immunogenetic factors.
Finally, neutrophil activation is thought to be of pathogenetic relevance, particularly in the ocular type of the disease (7). Zincrepresents one of the most important regulatory trace elements in the cellular immune response mediated by lymphocytes activation (8, 9); the importance of this oligoelement in eliciting a normal immune response is confirmed by several observations of cellular impairment in hypozinchemic conditions such as restriction of dietary zinc, acrodermatitis enteropathica or chronic renal insufficiency in patients under haemodialitic treatment (10-13).
In the attempt to evaluate a possible involvement of zinc in the immunopathogenesis of Behcet's disease, we have analyzed zinc concentrations in plasma, serum and erythrocytes in a group of Italian Behcet patients. The patients were also tested for the lymphoprolipherative response to mitogens (Phytohaemagglutinin. Pokeweed Mitogen), alloantigens (by Mixed Lymphocytes Reaction) and HSY 1.
Materials and methods
Population
The study is based upon 11 Italian patients all affected with the ocular type of BO and diagnosed as complete (n = 8) and incomplete (n = 3) according to the clinical criteria proposed by the Behcet's Disease Research Comittee of the Japanese Ministry of Health and Welfare (1). It was possible to reexaminc, 3 to 5 months later, 5 of the 11 BD patients. At the time of the study 4 BD patients were under therapy (all with steroids). Blood samples were also obtained from 24 volunteer healthy subjects.
Zinc concentration
Plasma. serum and erythrocyte zinc concentration was determined by flame atomic absorption spectrophotometry (Perkin Elmer mod. 1100). Venous blood samples were drawn into disposable plastic zinc-free syringes and transferred into plastic test tubes with zinc-free heparin for plasma and red blood cell separation.
Blood samples were separated within four hours of collection and plasma and sera showing haemolysis were discarded.The frozen sera and plasma were analyzed after I:10 dilution of the sample with deionized water. For erythrocytic zinc evaluation 0.5 ml of centrifuged red blood cells for each sample were haemolyzed with cold deionized water to reach a haemolysate dilution of 1:100.The zinc concentration was determined using an analytical calibration curve and expressed as µmol/L: the use of the background corrector was necessary. The merits of the analytical procedures were the following: 1) relative standard deviation: 3.5% (1.5 µmol/L). maximum 5% at the lowest concentrations observed and minimum 1% at Lhe highest concentrations observed); 2) limit of detection: 0.1 µmol/L (95%); 3) recovery: 94-101%.
Blastogenic response
Peripheral blood lymphocytes were separated in Lymphoprep (Nycomed AS, Norway), washed in PBS and resuspended at a concentration of 1 x 106/ml in Iscove medium (Gibco) supplemented with 10% inactivated AB pool sera. Lymphocyte cultures were made in 3072 Falcon microtiter plates using 0.1 ml of lymphocytic suspension. For mitogenic responses, preliminary experiments established the optimal range of concentrations for Phytohaemagglutinin P (Difco) and Pokewe·cd Mitogen (Gibco). For MLR. lymphocytes from 10 healthy subjects were inactivated by irradiation (4500 rads), pooled and used as stimulating cells; 1 x 105 responding lymphocytes and the same number of stimulating cells were cultured together in 0.2 ml medium.
The specific lymphoproliferative response Lo HSV1 was assessed by adding 0.1 ml of medium containing 45 mg/L of virus-purified proteins (14). Control cultures were prepared in the presence of an equal amount of cellular proteins obtained from the Hep-2 monolayer cell line in which virus is routinally grown. All cultures were performed in triplicate. Eighteen hours before harvesting, I µCi 3H-thymidine (Amersham-England, spec. act.: 2 Ci/mmol) was added to each well. The results were expressed in stimulation indices (S.I. = cpm stimulated cultures/cpm unstimulated cultures).
Statistical analysis was performed using the Mann-Whitney U test, one-tailed or two-tailed as appropriate, and linear regression analysis.
Result
Zinc concentration in BD patients and healthy controls are reported in Table I. Statistically significant increases were observed in BD patients, compared to controls, in plasma (p < 0.05) and erythrocytes (p < 0.02) (two-tailed Mann-Whitney U test) while serum didn't reach statistical significance. A further analysis performed on erythrocytes several months later, confirmed the stability of the zinc levels in patients (Table 2). The comparison between serum and plasma zinc levels within the two groups didn't show any significant difference: the slight differences observed could be merely due to the smaller size of the sample.
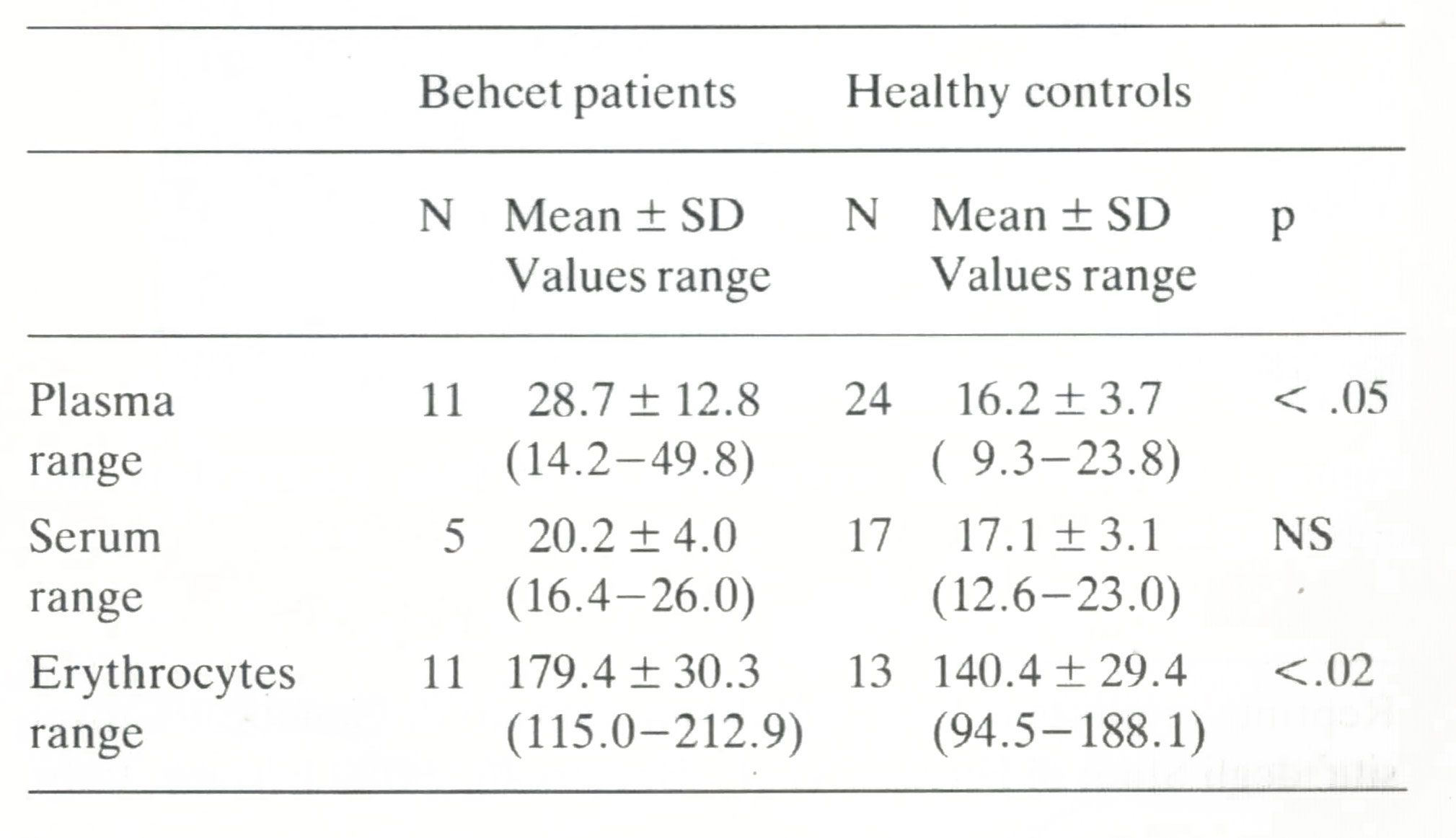
Table 1. Plasma. serum and erythrocytes zinc concentration in Behcet patientsand healthy controls. (Value,are given in11mol/L)

Table 2. Erythrocyte zinc level of 5 Behcet patients observed at different times. (Values are given in µmol/L)
Result
The patients' lymphoprolipherative responses to PHA, PWM, and alloantigens do not significantly differ from those observed in healthy controls (see Table 3); on the other hand, we were able to confirm the reported (15-17) impairment of the blastogenic response to the HSV1 antigen (p < 0.001); one-tailed Mann-Whitney U test (Table 3). Blastogenic responses obtained in autologous and in AB pool serum didn't show statistically significant differences (data not shown). No correlation was found in BD patients between zinc concentration and the impaired response to HSV1 antigen (r = .0219 and p = n.s.).
The patients under corticosteroid therapy didn't show levels of zinc statistically different from those of untreated patients.
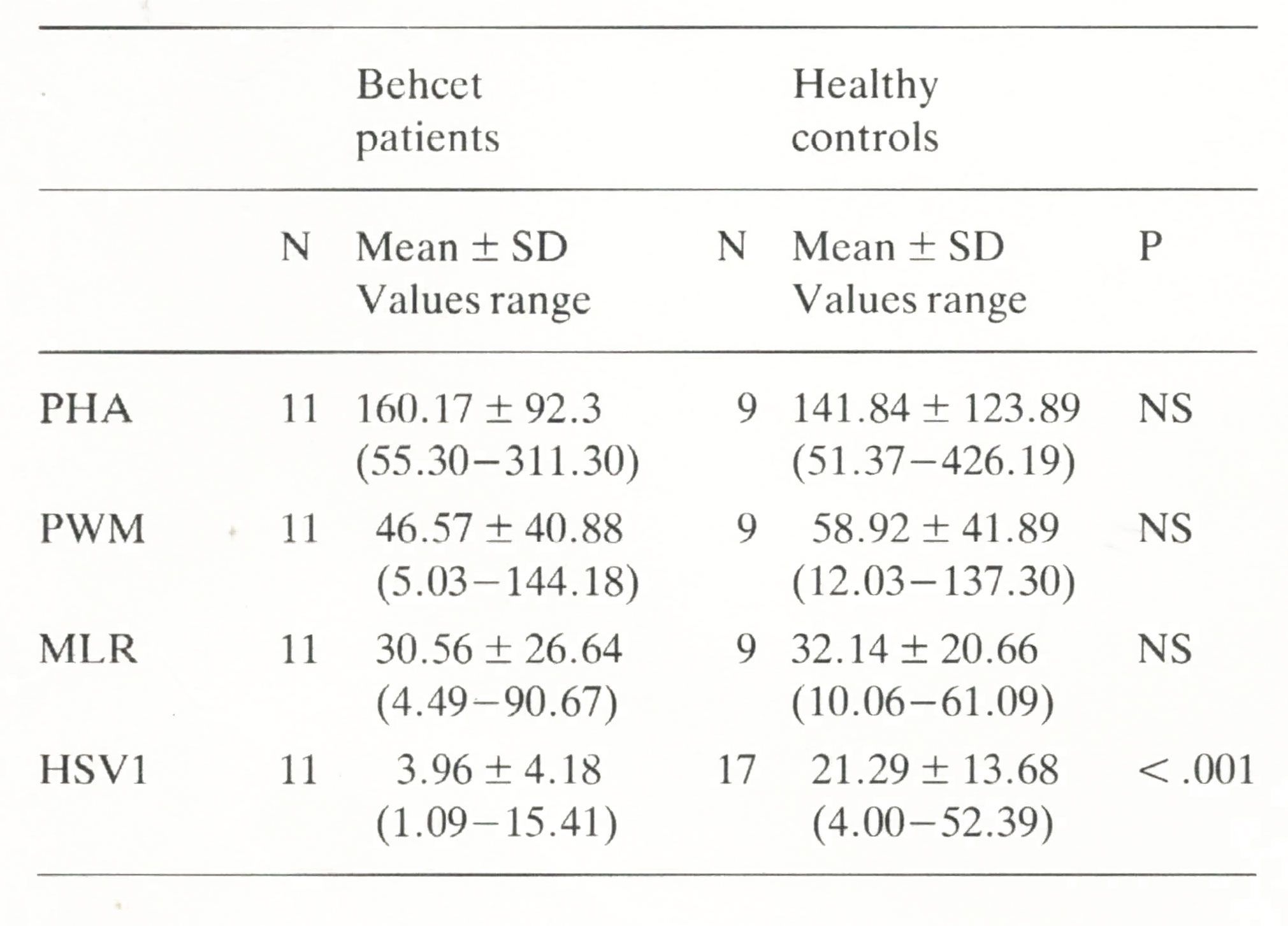
Table 3. Responses to PHA. PWM. MLR and HSV1 in Behcet patients and healthy controls expressed as stimulation indices. (Values arc given in S. I.)
Discussion
Zinc is one of the most important regulatory oligoelements in cellular immune response, effecting mainly T-lymphocytes, but also B-lymphocytcs and phagocytes.The impairment of cellular response as a consequence of low zinc levels and the restoring of normal cellular immune capacity following dietary treatments has been reported in various studies, confirming the role of this element in the immune system regulation (10, 13). On the other hand, very little is known about the biological effect of increased zinc levels; up to now, sporadic observations such as familial hyperzinchemia (18) have been the only available data, with no investigations of possible effects on the cellular response.
In the present study we have observed a significant increase of zinc levels in plasma and erythrocytes of patients affected by BD. Our reference ranges, for serum and plasma, resulted higher than those generally reported (19), however these data are in agreement with those previously found in our population (13). Two out of the five patients examined for serum zinc concentration showed levels above the normal range; however the results didn't reach statistical significance, probably due to the small size of the sample.
The finding of high intraerythrocytic zinc levels suggests a stable hyperzinchemic condition in these patients, because of the longlife span of erythrocytes (about 120 days). High zinc levels were also confirmed in further analyses performed at different times, thus excluding a temporary imbalance.
Inflammatory processes seem to influence serum zinc levels. In fact, in many chronic inflammatory diseases, such as Rheumatoid Arthritis, low serum zinc level is a common finding (20) and it is worth noticing that the fall in zinc concentration correlates with ESR, a good indicator of the precence of a phlogistic disease. Zinc seems to be lowered in serum because of its uptake in the inflamed tissues where interleukins may induce the increased synthesis of metal-binding proteins which serve a cellular protective function against free oxygen radicals (20). Surprisingly, in BD, a phlogistic disease considered by many authors to be connective tissue disorder just like RA, there was found not a decrease in zinc level but a significant increase.
Further investigation is needed in order to explain these data; however, it is tempting to speculate that this quite specific imbalance in BD could play an etiopathogenetic role. We speculate that zinc is in creased because it cannot be utilized in BD patients in modulating the immune response and this incapability is reflected in the chronic tissue damage.
In order to support this hypothesis the origin of zinc imbalance should be tested by examination of cell lines from BD patients. Furthermore, it could be help ful to investigate the increase in the zinc intracellular level in BD neutrophils, which in our opinion arc the most promising candidates for the pathogenesis of the tissue damage in the ocular type of BD (7).
Acknowledgements
This work was supported by C.N.R. "Progetto finalizzato ingegneria genetica e basi molecolari delle Malattie Ereditarie" 87.00846.51. We wish to thank Prof. E. Cassai for providing purified virus proteins.
References DA SISTEMEARE
-
Shimizu,T. ( 1974) Behcet's disease. Jpn. J. OphTaLmol. 18,291
-
Lehner. T., Losito A. & Williams, G.D. (1979) Cryoglobulins in Behcet's syndrome and recurrent oral ulceration: assay by laser nephelometry. CliN. exp. Immunol. 38, 436-438
-
ValEsini, G., PiveTTi Pezzi. P., Mastrandrea. F., Moncada A., Cuomo M. & Natali P.G. (1985) Evaluation ofTcell subsets in BEhcEt's syndrome uSing anti-Tcell monoclonal antibodies. CliN. exp. Immunol. 60. 55-58
-
Efthimiou J., Harikumar M.K., Knight. R.A. & Snaith M.L. (1984) Inappropriate peripheral blood lymphocyte responses to herpes viruses in patients with Behcet's syndrome. IMMUNOLOGY LeTTers 8. 317-318
-
Ohno. S., Anasuma T., SugiurA S., Wakisaka A. & Aizawa M. (1978) HLA Bw51 and Behcet's disease. JAMA 240. 529
-
Baricordi 0. R., Sensi A., Pivetti-Pezzi. P., Perrone S., Balboni A., Catarinelli G., Filippi F., Melchiorri L., Moncada A. & Mattiuz P.L. (1986) Behcet's disease associated with B51 and DRw52 antigens in Italians. Hum. immunol. /7,297-301
-
Niwa Y., Miyaka S., Sakane T., Shingu M. & Yokoyama M. (1982) Auto-oxidative damage in Behcet's disease-endotelial cell damage following the elevated oxigen radicals generated by stimulated neutrophils. Clin. exp. immunol. 49. 247-255
-
bach J.f.{1981) The multi-faceted zinc dependency of the immune system. immunology Today 2, 225
-
Salomons, N.W. (1982) Biological availability of zinc in humans. Am. J. Clin. Nutr. 35. 1048-1075
-
Prasad A.A. (1986) Effect of trace element imbalance in human disease. acta Pharmacol. Toxicol. 59. VII. 94- 103
-
Levander. O.A. (1986) Progress in establishing human trace elements requirements: selenium, zinc and copper. acta Pharmacol. Toxicol. 59. Vil, 83-89
-
Zumkley H., Zidek W. & Bertram H.P. (1984) Zinc substitution in renal insufficiency. Trace elem. Med. 1. 43-45
-
Gilli P., Baricordi 0., Fagioli F., de Paoli Vitali E., Malacarne F., Scanavini L., Mattiuz P.L. & Farinelli A. (1984) Plasma zincvconcentrations in patients with different degrees of chronic renal failure: possible role in lmphocyte function regulation. Trace Elem. Med. 1, 153-159
-
Ejercito P.M., Kieff E.D. & Roizman B. (1968) Characterization of Herpes simplex virus strains differing in their effect on social behaviour of infected cells. J. Gen. Virol. 2. 357-164
-
Bonass W.A., Stewart J.A., Chamberlain M.A. & Halliburton i.W. (1986) Molecular studies in Behcet's disease. In: Recent Advances in Behcet's Disease (T. Lehner and C.G. Hnrncs. eds.). p. 37. Royal Society of Medicine. London.
-
Denman A.M., Fialkow P.J., Pelton B.K., Salon A.C., Appleford D.J. & Gilchrist C. (1980) Lymphocyte abnormalities in Behcet's syndrome. Clin. exp. Immunol. 42, 175-178
-
Eglin P.P., Lehner T. & Subak-Shape J.H.(1982) Detection of RNA complementary to Herpes Simplex Virus in mononuclear cells from patients with Behcet's syndrome and recurrent oral ulcers. Lancet II. 1356
-
Smith J.C., Jr., Zeller J.A., Brown. E. D. & Ong S.C.(1976) Elevated plasma zinc: a heritable anomaly. Science 193, 496-498
-
Halsted J.A., Smith J.C. & Irwin M.I. (1974) A conspectus of research on Zinc requirements of man. J. Nutrit. 104. 345-378
-
Munthe E., Aaseth J. & Jellum E. (1986) Trace elements and rheumatoid arthritis (RA). Pathogenetic and therapeutic aspects. Acta Pharmacol. Toxicol. 59. Vil. 365-373